Product Code : 2D-BP-NN-CU
Black phosphorus is the thermodynamically stable form of phosphorus at room temperature and pressure, with a heat of formation of −39.3 kJ/mol (relative to white phosphorus which is defined as the standard state). It was first synthesized by heating white phosphorus under high pressures (12,000 atmospheres) in 1914. Black Phosphorus is a layered semiconducting material similar in appearance to graphite with numerous uses in optoelectronic, semiconductor, and photovoltaic applications. In a two-dimensional form, black phosphorus is known as Phosphorene and has similar properties to other 2D semiconductor materials such as graphene. Black phosphorus has an orthorhombic pleated honeycomb structure and is the least reactive allotrope, a result of its lattice of interlinked six-membered rings where each atom is bonded to three other atoms. In this structure, each phosphorus atom has five outer shell electrons. Black and red phosphorus can also take a cubic crystal lattice structure. The first high-pressure synthesis of black phosphorus crystals was made by the Nobel prize winner Percy Williams Bridgman in 1914. Metal salts catalyze the synthesis of black phosphorus
Please contact us if you need customized services. We will contact you with the price and availability in 24 hours.
| Product | Product Code | Purity | Size | Contact Us |
Product Information
Black phosphorus is the thermodynamically stable form of phosphorus at room temperature and pressure, with a heat of formation of −39.3 kJ/mol (relative to white phosphorus which is defined as the standard state). It was first synthesized by heating white phosphorus under high pressures (12,000 atmospheres) in 1914. Black Phosphorus is a layered semiconducting material similar in appearance to graphite with numerous uses in optoelectronic, semiconductor, and photovoltaic applications. In a two-dimensional form, black phosphorus is known as Phosphorene and has similar properties to other 2D semiconductor materials such as graphene. Black phosphorus has an orthorhombic pleated honeycomb structure and is the least reactive allotrope, a result of its lattice of interlinked six-membered rings where each atom is bonded to three other atoms. In this structure, each phosphorus atom has five outer shell electrons. Black and red phosphorus can also take a cubic crystal lattice structure. The first high-pressure synthesis of black phosphorus crystals was made by the Nobel prize winner Percy Williams Bridgman in 1914. Metal salts catalyze the synthesis of black phosphorus
The similarities to graphite also include the possibility of scotch-tape delamination (exfoliation), resulting in phosphorene, a graphene-like 2D material with excellent charge transport properties, thermal transport properties and optical properties. Distinguishing features of scientific interest include a thickness dependent band-gap, which is not found in graphene. This, combined with a high on/off ratio of ~105 makes phosphorene a promising candidate for field-effect transistors (FETs). The tunable bandgap also suggests promising applications in mid-infrared photodetectors and LEDs. Exfoliated black phosphorus sublimes at 400 °C in vacuum. It gradually oxidizes when exposed to water in the presence of oxygen, which is a concern when contemplating it as a material for the manufacture of transistors, for example. Exfoliated black phosphorus is an emerging anode material in the battery community, showing high stability and lithium storage.
Synonyms
Phosphorene, BP, (5N) 99.999% Phosphorus Black Crystal
Black Phosphorus Specification
Size:customized
Purity: 99%(2N), 99.9%(3N), 99.99%(4N), 99.999%(5N).
Per your request or drawing
We can customized as required
Properties(Theoretical)
| Atomic number | 15 |
| Atomic radius - Goldschmidt ( nm ) | 0 |
| Molecular Weight | 30.97 |
| Appearance | Gray to black crystals |
| Electronic structure | Ne3s²p³ |
| Ionisation potential ( No./eV ) | 3 / 30.18 |
| Ionisation potential ( No./eV ) | 4 / 51.37 |
| Ionisation potential ( No./eV ) | 6 / 220.43 |
| Ionisation potential ( No./eV ) | 1 / 10.49 |
| Ionisation potential ( No./eV ) | 2 / 19.72 |
| Ionisation potential ( No./eV ) | 5 / 65.02 |
| Thermal neutron absorption cross-section ( Barns ) | 0.19 |
| Valences shown | 4 , ±3, 5 |
| Boiling Point | N/A |
| Density | 2.34 g/cm3 |
| Melting Point | 416 °C |
| Solubility in H2O | 0.3 g/l (20 °C) |
| Exact Mass | 30.973762 g/mol |
| Monoisotopic Mass | 30.973762 g/mol |
Properties of some allotropes of phosphorus
| Form | white(α) | white(β) | violet | black |
| Symmetry | Body-centred cubic | Triclinic | Monoclinic | Orthorhombic |
| Pearson symbol | aP24 | mP84 | oS8 | |
| Space group | I43m | P1 No.2 | P2/c No.13 | Cmca No.64 |
| Density (g/cm3) | 1.828 | 1.88 | 2.36 | 2.69 |
| Bandgap (eV) | 2.1 | 1.5 | 0.34 | |
| Refractive index | 1.8244 | 2.6 | 2.4 |
2D Black Phosphorus Properties
Like other van der Waals 2-dimensional materials such as MoS2, the optical, electronic, and mechanical properties of phosphorene differ from that of the bulk state due to combinations of factors. These include:
High surface-to-volume ratio
Out-of-plane charge carrier confinement
No interlayer interactions
Increased Coulomb interaction between charge carriers (reduced dielectric screening)
A hole mobility up to ~1000 cm2V-1s-1 has been measured in few-layer phosphorene in a FET structure . The anisotropy of carrier mobility travelling along the AC and ZZ directions has also been demonstrated
Specifically, after exfoliation of black phosphorus crystals or powder, black phosphorus typically has the following properties:
Orthorhombic C puckered honeycomb structure
0.3eV ~ 1.5 eV thickness-dependent bandgap
High charge carrier mobility ~1000 cm2V-1s-1
Thermal conductivity of 86 (34) Wm-1K-1 in ZZ (AC) direction (few-layer)
On/off ratio of 105
Black phosphorus powder can be combined with liquid-phase exfoliation or further chemical modification to control the physical and electronic properties of the resultant semiconductor material. Liquid-phase exfoliation is often used to create black phosphorus quantum dots. Black phosphorus quantum dots have an average size of 4.9 nm and a thickness of 1.9 nm.
Band Gap of Phosphorene
Black phosphorene has a thickness-dependent direct band gap, ranging from 1.88 eV (for a single monolayer) to 0.3 eV (for the bulk material).
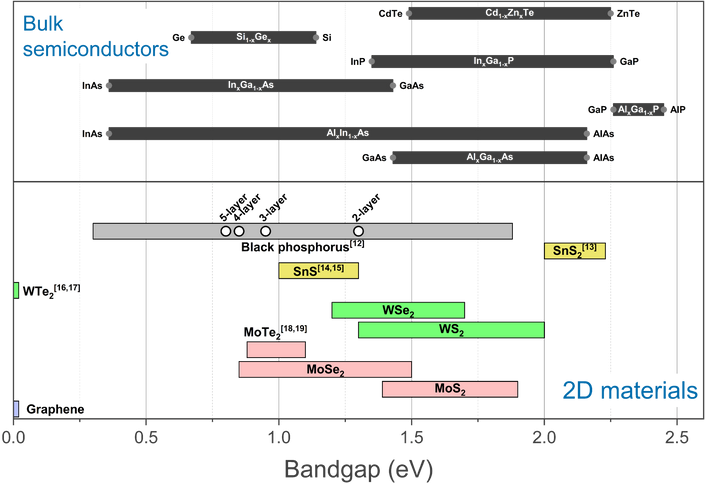
A comparison between the band gaps of several 2-dimensional materials is shown below, along with those of conventional bulk semiconductors. The wide tunability of the phosphorene bandgap by changing the sample thickness makes it attractive for a number of applications.
Bandgap energies of 2D materials and conventional bulk semiconductors
Processing Black Phosphorus Phosphorene monolayers and multilayers can be produced from bulk black phosphorus through either mechanical (scotch-tape method) or liquid exfoliation. CVD growth has recently been reported for few-layer thicknesses with areas of a few square microns [29]. Black phosphorus degrades under ambient conditions. Studies show that water alone does not result in its degradation. However, oxygen plays an important role in its degradation by inducing changes in its electronic structure.
Applications of Black Phosphorus
Due to its unique properties, exfoliated monolayer and few-layer black phosphorus has potential for a wide range of applications in electronics and optoelectronics. Applications that have been suggested (or are currently being investigated) include in LEDs, photodetectors, supercapacitors, superconductors and memory devices. Black phosphorus powder can be used to synthesis black phosphorus quantum dots (BPQDs). The properties of BPQDs make them well suited for the development of thermoelectric devices, sensors, LEDs, OPVs, and energy storage systems.
Photovoltaics and solar cells
The photovoltaic effect has been observed in few-layer black phosphorus . With thicker samples having a bandgap smaller than that of silicon, it could be used to harvest the NIR-IR region of the solar spectrum that silicon cannot access. While the observed external quantum efficiencies observed so far are small (<1%), it has been predicted that a modified phosphorene structure could reach efficiencies of 20% .
Gas sensors
Phosphorene is an interesting prospect for chemical sensing due to its large surface-to-volume ratio and the presence of a lone electron pair on each atom. A NO2 sensor has been demonstrated with a sensitivity of 20 parts per billion in air , A theoretical study has suggested that single molecule sensing may be possible with such sensors
Thermoelectric applications
Phosphorene combines low thermal and high electrical conductivity, making it suitable for thermoelectric applications.
Flexible memory devices
Black phosphorus quantum dots have the potential to be used as the active layer in flexible memory devices. They have exhibited a non-volatile, re-writable memory effect with high on/off current ratios (more than 6.0 × 104).
Energy storage and battery electrodes
Phosphorene combines significant, reversible charge-storage capacity with small volume change and good electrical conductivity. This combination of properties makes it good candidate for energy storage applications. Phosphorene has therefore been proposed as an anode material for Li-ion batteries, with lithium diffusion expected to be orders of magnitude faster than in other 2D materials . Structural engineering, incorporation into heterostructures with other 2D materials, and addition of defect states is expected to improve lithium diffusion rates and binding energies.
Next generation batteries
Few-layer black phosphorus may also find application in future sodium ion batteries (the expected replacement for lithium ion) . The large interlayer spacing allows for the diffusion of the lar
Field-effect transistors for electronic devices
Field-effect transistors (FETs) are the most studied of phosphorene’s potential applications, with many theoretical and experimental studies carried out over the last 5 years. The attractive FET characteristics of relatively high on/off ratio and good charge carrier mobility along with a high conductivity should ensure fast switching with high efficiency and error-free logic.
Photodetectors in communication networks
Phosphorene’s direct bandgap is tunable (between 0.3 eV to 1.88 eV) by changing the number of stacked layers. This makes it optically active in the red to NIR spectrum and has allowed the fabrication of visible to NIR photodetectors . This region of the spectrum is important for optical fibre networks and suggests that phosphorene could play a role in future communication networks.
Packing of Black Phosphorus
Standard Packing:
Typical bulk packaging includes palletized plastic 5 gallon/25 kg. pails, fiber and steel drums to 1 ton super sacks in full container (FCL) or truck load (T/L) quantities. Research and sample quantities and hygroscopic, oxidizing or other air sensitive materials may be packaged under argon or vacuum. Solutions are packaged in polypropylene, plastic or glass jars up to palletized 440 gallon liquid totes Special package is available on request.
ATTs’ High Purity Black Phosphorus is carefully handled to minimize damage during storage and transportation and to preserve the quality of our products in their original condition.
Chemical Identifiers
| Linear Formula | P |
| CAS | 7723-14-0 |
| MDL Number | MFCD00011435 |
| EC No. | 231-768-7 |
| Pubchem CID | 5462309 |
| IUPAC Name | phosphorus |
| SMILES | [P] |
| InchI Identifier | InChI=1S/P |
| InchI Key | OAICVXFJPJFONN-UHFFFAOYSA-N |